Appendix 1: Further background
A1.1 This appendix provides further background to the Report.
A1.2 The first part gives information about the Panel’s analysis of the prescribing and administration of drugs at Gosport War Memorial Hospital (‘the hospital’). This information complements and explains the number of deaths described in Chapter 2 and in Table 1.
A1.3 The second part describes the General Medical Council (GMC) and its processes. This information may be helpful in understanding Chapter 6 of the Report.
The Panel’s analysis: further information
A1.4 The Panel’s Terms of Reference (see Appendix 3) require the Panel through its Report to provide an overview of the information reviewed and to illustrate how the information disclosed adds to public understanding of events at the hospital and their aftermath. In order to do so, the Panel has reviewed the documents secured in the way described in Chapter 11 and overseen maximum possible public disclosure via this Report and the accompanying website. In addition, the Panel has been able to consider the clinical records and death certificates for those patients who died at the hospital between 1987 and 2001.
A1.5 The Panel’s Report brings together its review of this range of documents. Chapter 2 explains the Panel’s resulting analysis of the pattern of prescribing and administering drugs. The Panel started by reviewing the information available for the 163 patients in the Initial Group. The picture emerging led the Panel to examine a Wider Group of 1,043 patients, where sufficient records were available. Chapter 2 highlights the number of deaths of patients from this group where opioids were prescribed and administered without appropriate clinical indication.
A1.6 The analysis of the Wider Group, as well as the analysis of the Initial Group, is clearly fundamental to the Panel’s Report. Accordingly, it may be helpful to provide further information about how the analysis of the Wider Group relates to the analysis of the Initial Group. In a random selection of 30 patients from the Wider Group, the Panel performed an analysis identical to that completed for the Initial Group. The Panel found that 23 of these 30 patients (77%) received continuous diamorphine through a syringe driver. Of these 23 patients, nine of them had not previously received diamorphine orally or another opioid such as fentanyl before the diamorphine was commenced through a syringe driver. These nine patients were therefore opioid naïve.
A1.7 Figure 12 shows the starting dose of diamorphine for these 23 patients. As in the Initial Group, the diamorphine starting dose is excessive in the majority of patients in this sample.
Figure 12: Starting dose of diamorphine for 23 patients from the Wider Group
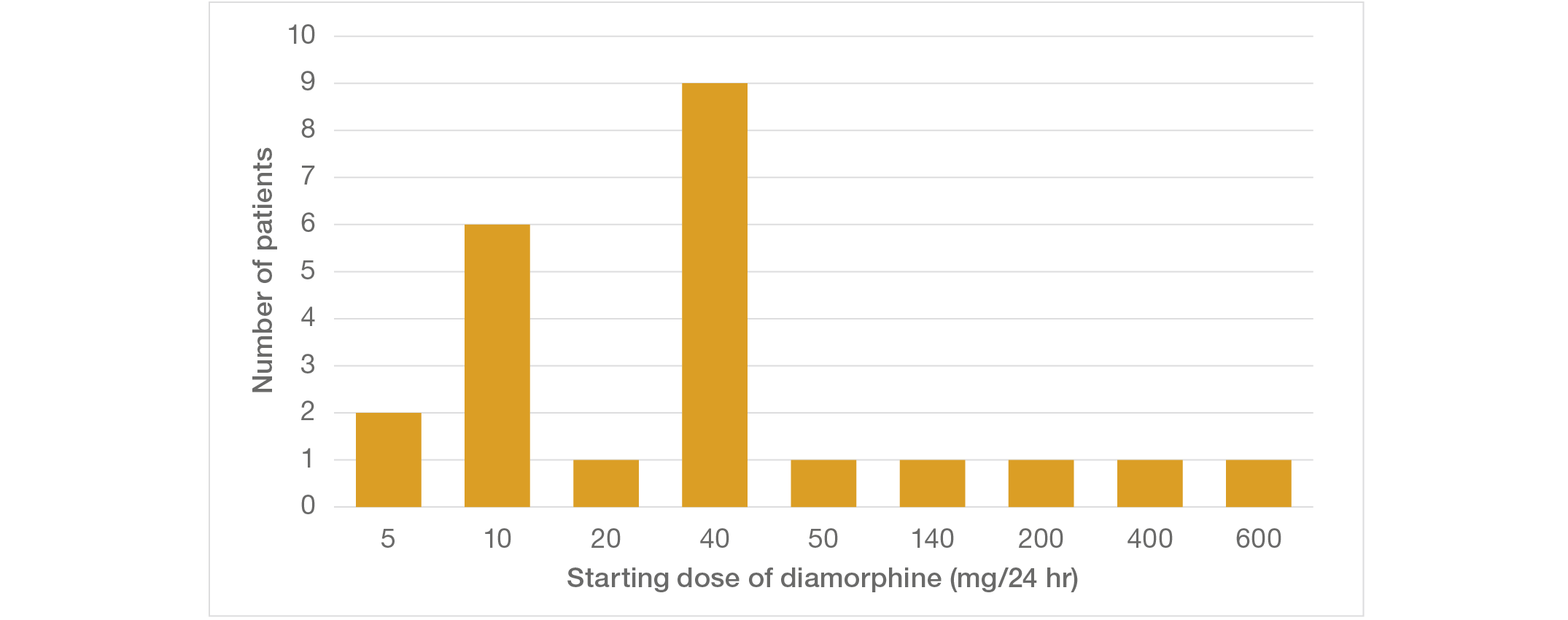
A1.8 Six of the 23 patients received oral morphine before the opioid dose was escalated to continuous and subcutaneous diamorphine by syringe driver. To maintain dose equivalence, the dose of subcutaneous diamorphine should be between 33% and 50% of the oral morphine dose. From this sample, one patient received a dose of diamorphine eight times the final dose of oral morphine. These findings again confirm that the dosages of opioids used at the hospital far exceeded normal conventional practice.
A1.9 This further information is illustrative of the detailed work the Panel conducted in relation to the 30 patients in the Wider Group. The Panel has concluded that its examination of this group demonstrates that opioid use in this group showed the same features as those in the Initial Group of patients.