Chapter 2: Prescribing and administration of drugs and the deaths that resulted
The main findings from the Panel’s analysis of the documents
The Panel’s main findings from its analysis of the documents relating to the prescribing and administering of drugs are as follows:
- Finding One: Opioid usage without appropriate clinical indication
- Finding Two: Anticipatory prescribing with a wide range of doses
- Finding Three: Continuous opioid usage for patients admitted for rehabilitation or respite care
- Finding Four: Continuous opioids started at inappropriately high doses
- Finding Five: Opioids combined with other drugs in high doses
- Finding Six: Few patients survived long after starting continuous opioids
- Finding Seven: Prescription and administration of drugs contravened guidelines
- Finding Eight: Occurrence and certification of deaths.
These have been formulated through privileged access to medical records and supporting documentation granted to the Panel alone. As such, the process has not involved any other party.
Finding One: Opioid usage without appropriate clinical indication
The first finding, opioid usage without appropriate clinical indication, is the critical turning point on which the other findings, in this chapter and elsewhere, depend. As already noted, opioids are powerful drugs that bring significant benefits when used appropriately, but they carry commensurate risks.
The Panel looked at all the clinical records available for those 163 patients it initially knew about (see paragraph 2.3), to examine opioid usage. It collected information on the prescribing and administration of drugs, together with clinical information. In 58 cases, the clinical records, or key parts of them, could not be found; in the remaining 105, the Panel looked at whether opioids were used without appropriate clinical indication, taking into account the clinical picture, previous use of analgesics, reasons for starting continuous opioids, escalation of dosage and the use of other medication and alternatives. In many, the clinical records were of poor quality, hampering the search for evidence. Nevertheless, for 71 patients there was evidence that opioids were used without appropriate clinical indication.
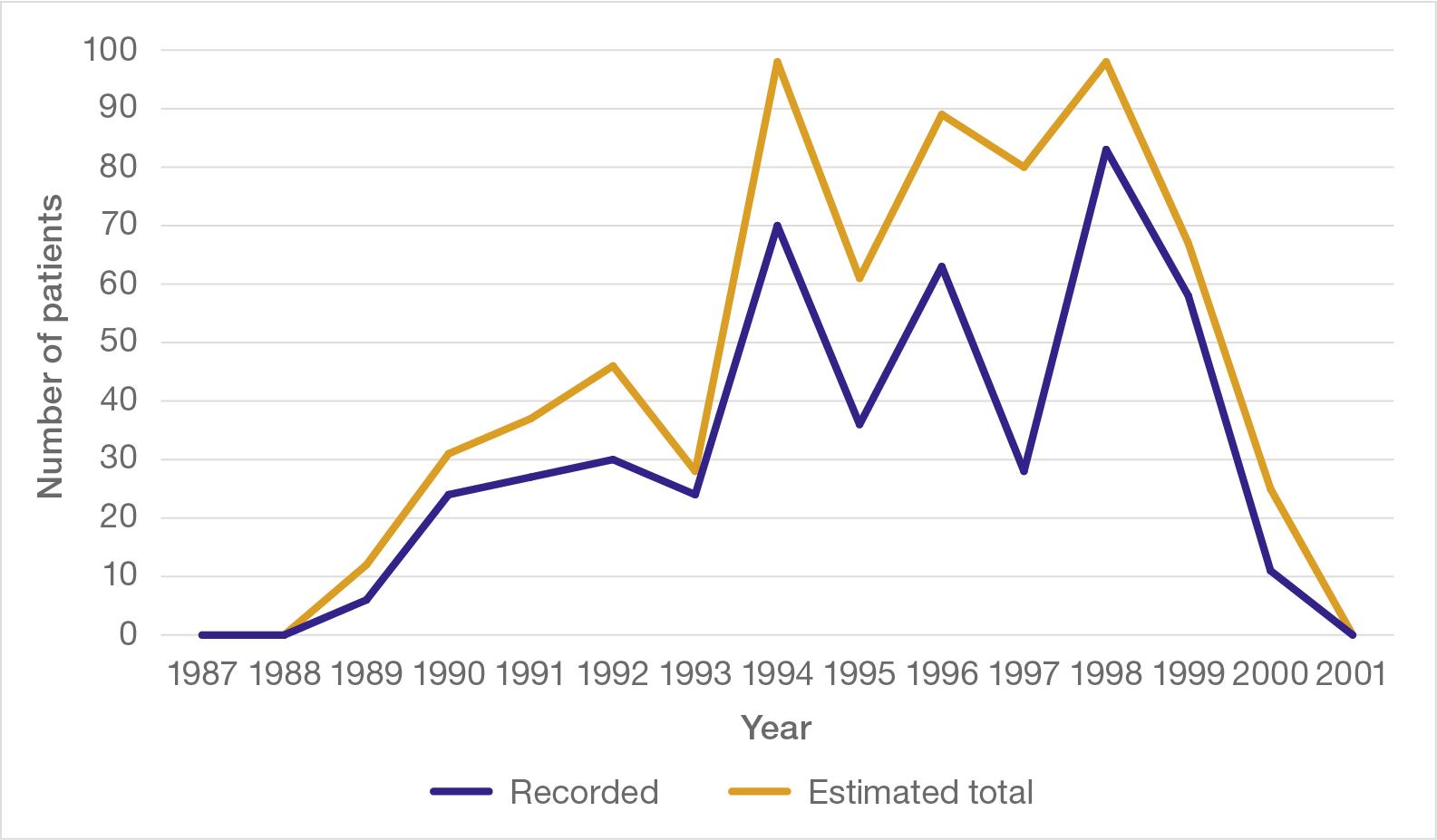
The starkness of this finding raised concern that patients other than those of whom the Panel was initially aware might also have been affected. It therefore sought the clinical records of the 2,024 patients known to have died in the hospital between 1987 and 2001, a period which appeared to cover the start and end of the pattern of opioid prescribing of concern. Hospital records for 1,564 of these patients were found, and it was considered whether opioids had been used without appropriate clinical indication.
In 1,043 of this Wider Group of patients there was sufficient information to form a view, although the Panel was again hampered by the poor quality of the clinical records. In 385 of these, there was evidence of opioid usage without appropriate clinical indication. A more detailed examination of the records of 30 of the Wider Group, described in Appendix 1, found that opioid use showed the same features as those in the Initial Group of patients.
In total, the Panel found evidence of opioid usage without appropriate clinical indication in 456 patients – that is, in 40% of the records that contained sufficient information. Taking into account the missing records, there were probably at least another 200 patients similarly affected but whose clinical notes were not found.
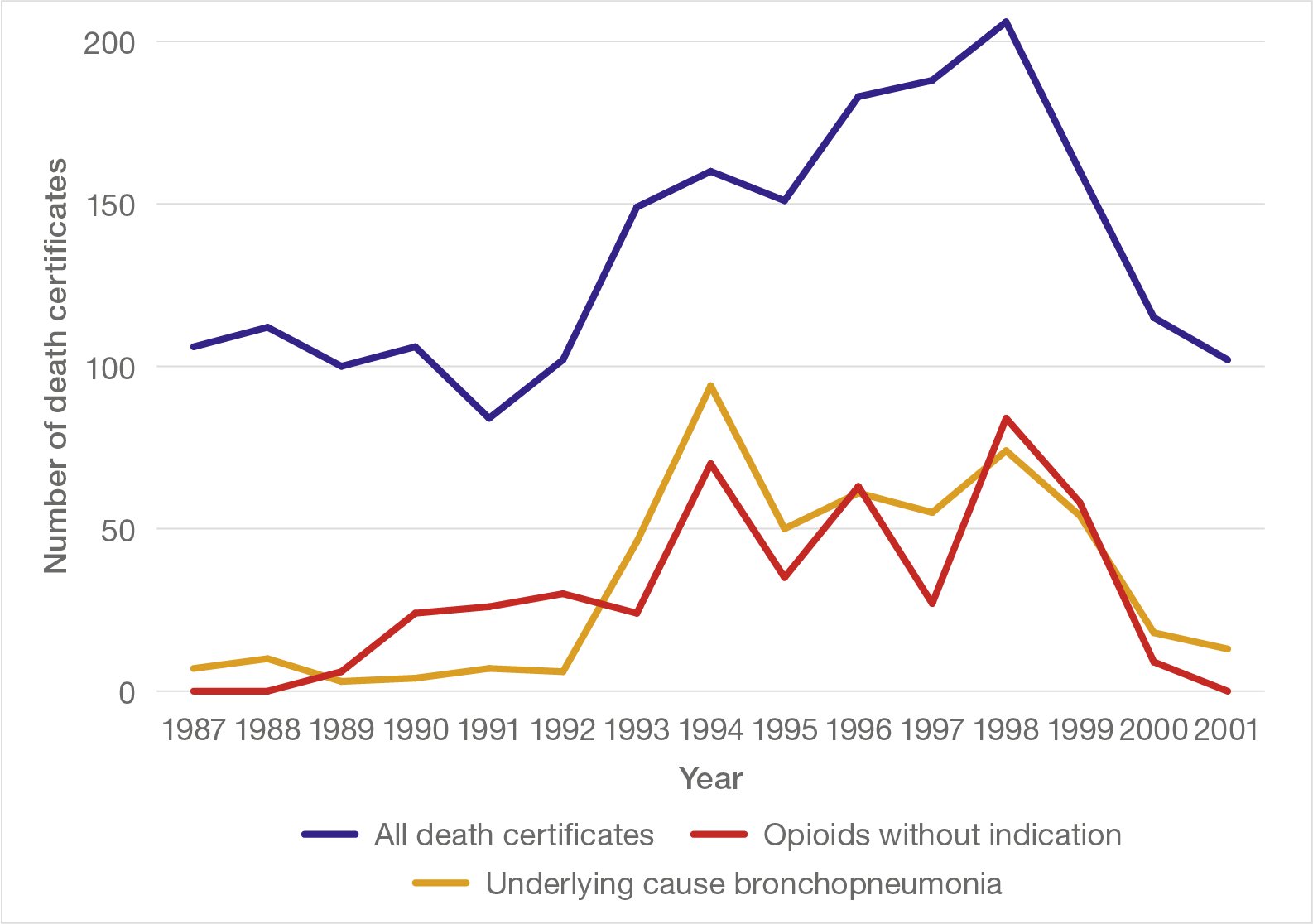
The occurrence of opioid usage without appropriate clinical indication followed a striking pattern over time (see Figure 2). There were no instances found in 1987 or 1988, but from 1989 the numbers rose strikingly. This was followed by an equally striking decline over 1999 and 2000, with no instances in 2001.
Figure 2: Opioid use without appropriate clinical indication, 1987 to 2001, numbers per year
In the great majority of patients, the opioid was diamorphine administered via a syringe driver, often in conjunction with other drugs, particularly midazolam and hyoscine, over the last few days of life. This corresponds closely with the concerns expressed by families.
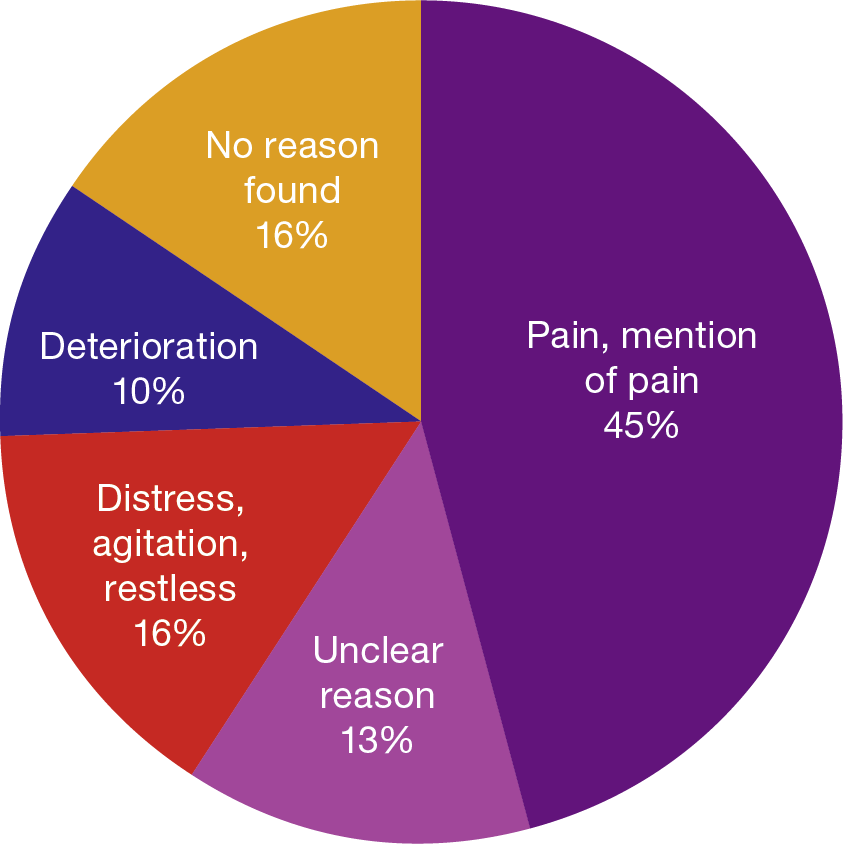
It is important to note that opioids, including diamorphine, are central to the management of acute or chronic pain, as set out in paragraph 2.12; occasionally opioids might be used for indications other than pain, such as breathlessness or cough. Only 45% of the Initial Group of patients with complete drug information had any suggestion of pain recorded in their notes (see Figure 3); in the majority of these, strong opioids would not have been clinically indicated as the first choice, or in the doses given.
The other stated reasons for diamorphine administration in the Initial Group would rarely, if ever, be regarded as appropriate indications, including deterioration, distress, restlessness and agitation. In 29%, no reason was found or no clear rationale was stated in the clinical records.
Figure 3: Recorded reason for administering diamorphine (97 patients in Initial Group given diamorphine)
Finding Two: Anticipatory prescribing with a wide range of doses
Anticipatory prescribing, or pre-prescribing, is the prescribing of a drug before it is necessary, so that it can be administered as soon as it is needed. When done appropriately, as part of a specified care plan with a defined reason to start and increase dosage within reasonable limits, it is a well-regarded part of end of life care. This is, however, very different from its use in patients admitted for a range of reasons other than end of life care, with no recorded decision on end of life care, and no recorded ‘triggers’ to start or to escalate dosage. There was no evidence in the documents seen by the Panel of the use of any guidance on those wards during the 1990s on the use of anticipatory prescribing or the need for careful controls.
More usually, opioids would be prescribed as a fixed dose to be given at regular intervals with, perhaps, additional amounts if required to control pain. The decision to administer any additional amounts would be made by a trained nurse and recorded in the clinical records with the reason they were necessary.
In contrast, the records show that practice at the hospital included anticipatory prescribing of diamorphine by syringe driver in a very wide dose range of either 20–100 mg per 24 hours or, more commonly, 20–200 mg per 24 hours, with no specified trigger for the start or escalation of dosage. In some, prescribing was done on the day of admission of patients not admitted for end of life care (see Table 2). This is contrary to all existing guidance at the time, including the Wessex guidelines and the BNF (1997). The records show that this was the reason that many patients did not receive opioids until several days after they had been prescribed.
Table 2: Inappropriate use of anticipatory prescribing of diamorphine on day of admission when not clinically indicated

The use of anticipatory prescribing of opioids before a patient requires end of life care carries significant risks and places unreasonable responsibility on nursing staff. First, as evidenced in some patient records, a change in the patient’s condition could be misinterpreted as a terminal event, prompting the start of a potentially lethal drug regime. Second, again as evidenced in some patient records, opioids already prescribed in this way could be used as an inappropriate response to a patient’s agitation or challenging behaviour. It is notable that in some cases the depressed consciousness that resulted from such inappropriate administration of opioids and other powerful sedatives was then ascribed, with no supporting evidence, to pathological changes such as stroke, and the drug regime was continued or increased.
The very wide dose range prescribed also placed additional undue responsibility on nursing staff who had to judge what dose to administer and when to increase it. The clinical notes did not include a record of what indications to use when making such a decision. Equally significantly, the nursing notes show little information about the reasons for escalation of the dose.
Finding Three: Continuous opioid usage for patients admitted for rehabilitation or respite care
The documents reviewed by the Panel show that the practice of pre-prescribing opioids extended to patients who had been admitted for rehabilitation or respite care.
While there may be reasons to consider anticipatory prescribing under carefully controlled conditions for end of life care, as noted in paragraph 2.106, this is very different from pre-prescribing opioid medication for patients who have been assessed as requiring rehabilitation or a period of respite care. The finding in clinical records that this happened repeatedly in patients admitted for rehabilitation or respite care was particularly surprising. These were patients, albeit frail, who had planned admissions but who were given powerful medication capable of suppressing consciousness and respiration.
Of the 163 Initial Group of patients in Table 1, there was sufficient information in the clinical records available to allow detailed analysis of prescribing, administration and reason for admission in 116 patients; 54 of these had been admitted for either respite care or rehabilitation. Diamorphine was prescribed for 49 (91%) of these patients, and administered in 47 (87%). A total of 35 (65%) received a combination of continuous diamorphine, midazolam and hyoscine.
The repeated finding of diamorphine usage in patients with a stroke was also of concern. Pain after a stroke is not usually of sufficient severity to warrant morphine or diamorphine; painful muscle spasm is common but usually managed with standard non-opioid analgesics or antispasmodics, with tricyclic antidepressants or antiepileptic therapy for less common pain of neurological origin. Yet some patients admitted to the hospital after a stroke received diamorphine, with minimal evidence in the clinical notes that these patients had severe pain. Others were given opioids and, when their consciousness was unsurprisingly reduced, diagnosed as having suffered a probable stroke. However, there was no record of any examination for neurological signs of a stroke.
Finding Four: Continuous opioids started at inappropriately high doses
Established guidance on the use of pain relief is clearly based on an incremental approach, in which less potent and risky drugs are tried first, with progression to the most potent continuous opioids only when necessary for severe intractable pain not controlled by other agents. When continuous opioids are used, they should not be the first line of treatment, and the dose should be carefully controlled. This is the basis of the WHO analgesic ‘ladder’ (see Figure 1) and the local Wessex guidelines.
The records show that diamorphine was often used without prior use of less potent analgesics, contrary to the guidance. Of the 97 patients in the Initial Group administered diamorphine, 23 (24%) had had no prior opioid of any description. Although the remainder had had some form of opioid, including oral morphine, modified release morphine or fentanyl, in many cases this had been given for too short a period for the effects to be assessed prior to escalating treatment to continuous diamorphine via a syringe driver.
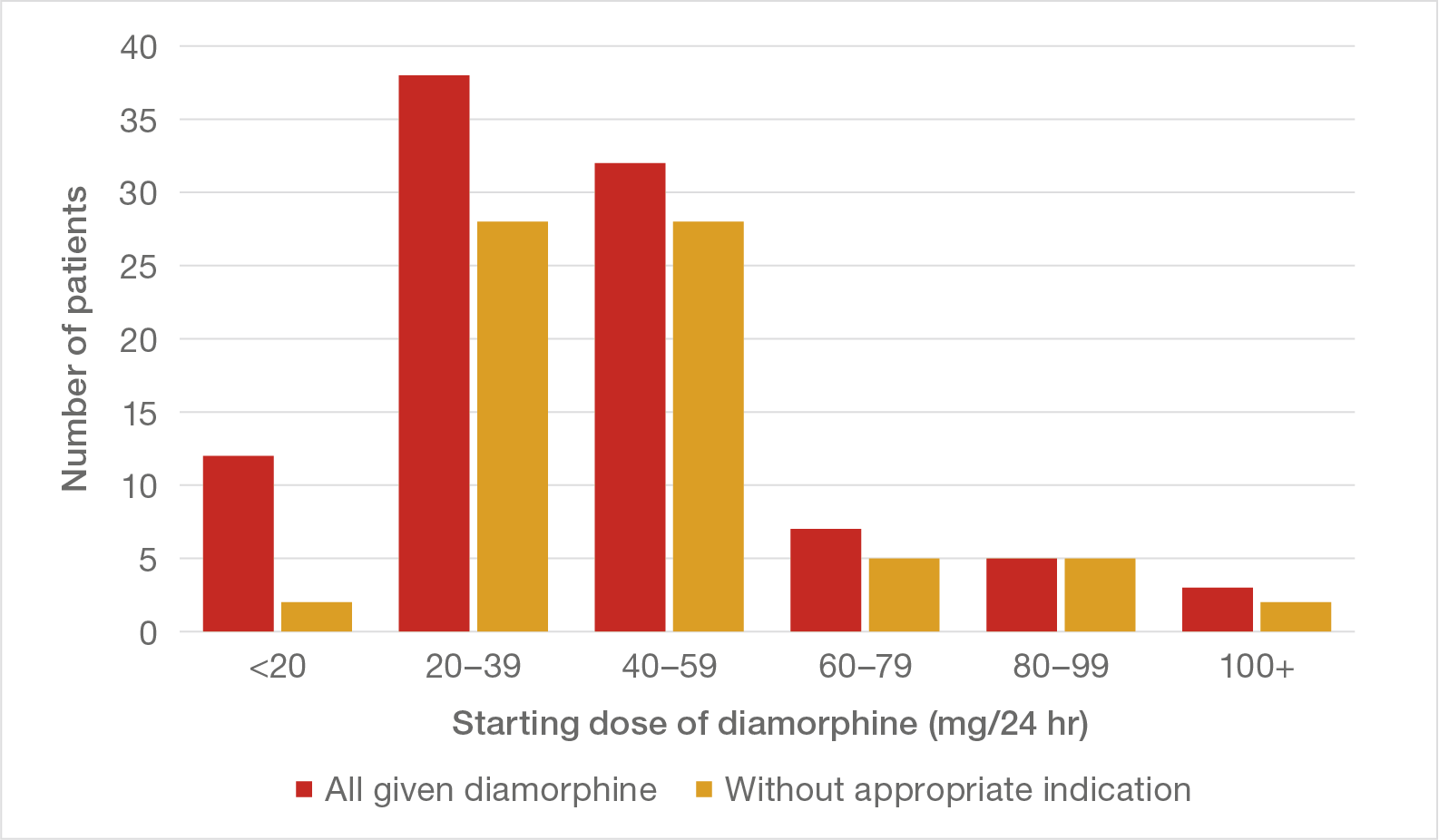
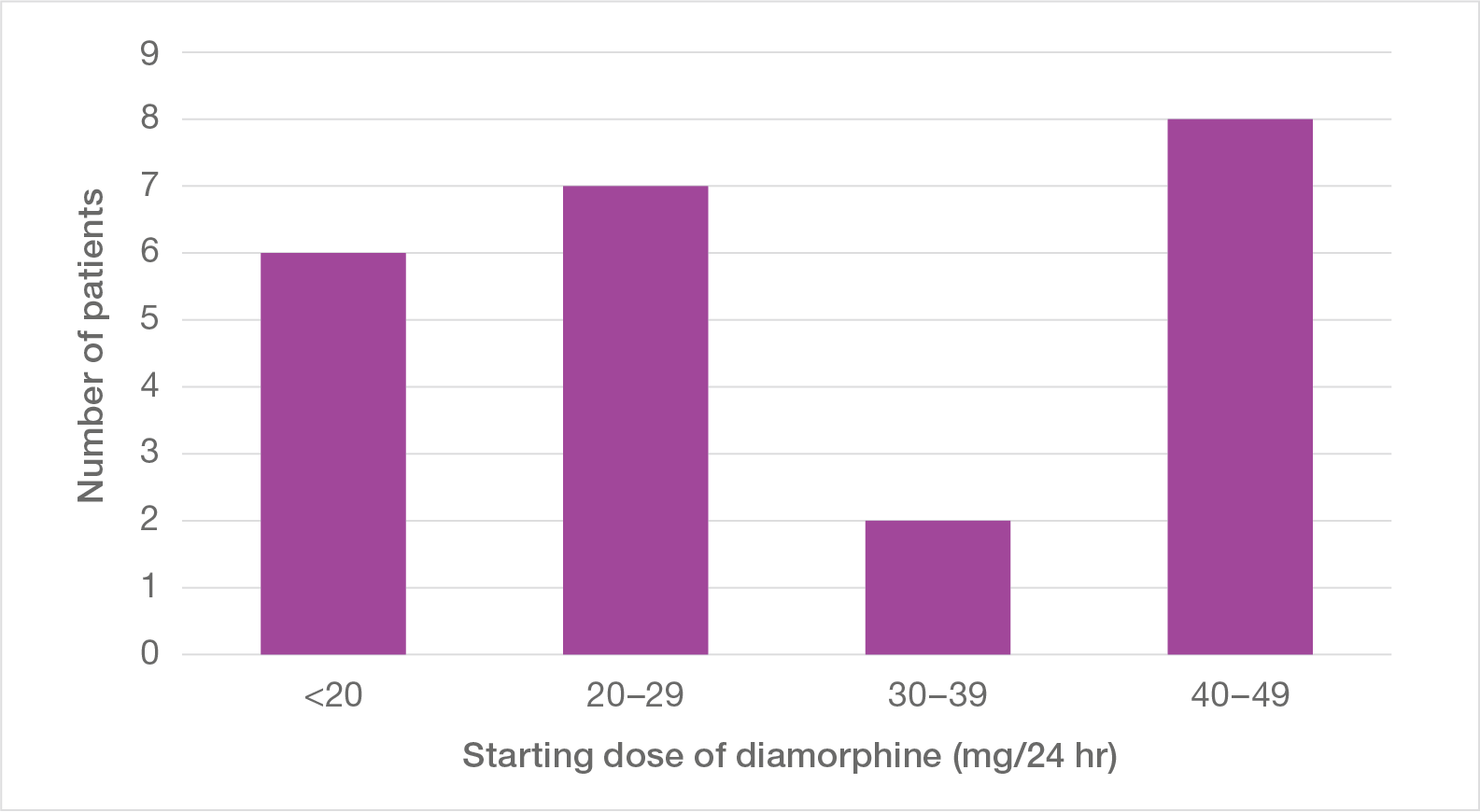
When continuous diamorphine was used, the initial dose was often high, most commonly 20 mg per 24 hours or 40 mg per 24 hours as shown in Figure 4. Diamorphine was commonly prescribed in a wide dose range with a lower limit of 20 mg per 24 hours, but it is notable that in almost half (48%) an initial dose of 40 mg per 24 hours or higher was selected. Although the range prescribed often went as high as 100 mg per 24 hours or even 200 mg per 24 hours, it is remarkable that so many patients were judged to require such a high initial dose. This is even more striking for those patients in whom there was no appropriate clinical indication for the diamorphine usage, with 57% receiving an initial dose of 40 mg per 24 hours or higher.
Figure 4: Starting dose of diamorphine in all patients given the drug in the Initial Group (97) and in those patients with evidence that diamorphine had been given without appropriate clinical indication
There are occasions when high levels of continuous opioids are necessary and appropriate, such as when patients in severe pain have developed a tolerance to the drug. This usually occurs when patients have been receiving continuous morphine or diamorphine as part of end of life care with an escalating dose carefully titrated against pain level and tolerance. There are, however, occasions when patients are switched from another opioid, such as oral morphine or fentanyl patches, and may need a higher initial dose as a result, but this is insufficient to explain the remarkably high starting doses in these patients. Figure 5 shows the starting dose of diamorphine for 23 patients who went straight to diamorphine with no preceding other opioid and were therefore particularly susceptible to the effects. A similar pattern is evident, with 8 of the 23 starting on 40 mg per 24 hours.
Figure 5: Starting dose of diamorphine in those who had had no prior form of opioid
Where a prior opioid had been used (70 patients), this had sometimes been for such a short period that there was neither time for its effect to be assessed nor for a tolerance to develop that might have justified a higher initial dose of continuous diamorphine. Some 23% of this group had received another form of opioid for only one day or less before being switched to continuous diamorphine.
In 40 patients who had been given oral morphine for longer, the records of drug administration were sufficiently complete to allow a comparison to be made between the last dose of oral morphine and the initial dose of diamorphine that followed (Figure 6). As the most relevant guidance (BNF) makes clear, parenteral diamorphine has approximately three times the potency of oral morphine preparations. To maintain equivalence when making this switch, the dose of diamorphine should have been one-third of the dose of oral morphine over a 24-hour period. No fewer than 35 (88%) patients in this group received an initial dose in excess of this and therefore outside the guidelines; in several cases the initial dose of diamorphine was four or more times larger.
Figure 6: Relationship between first dose of diamorphine and preceding dose of oral morphine
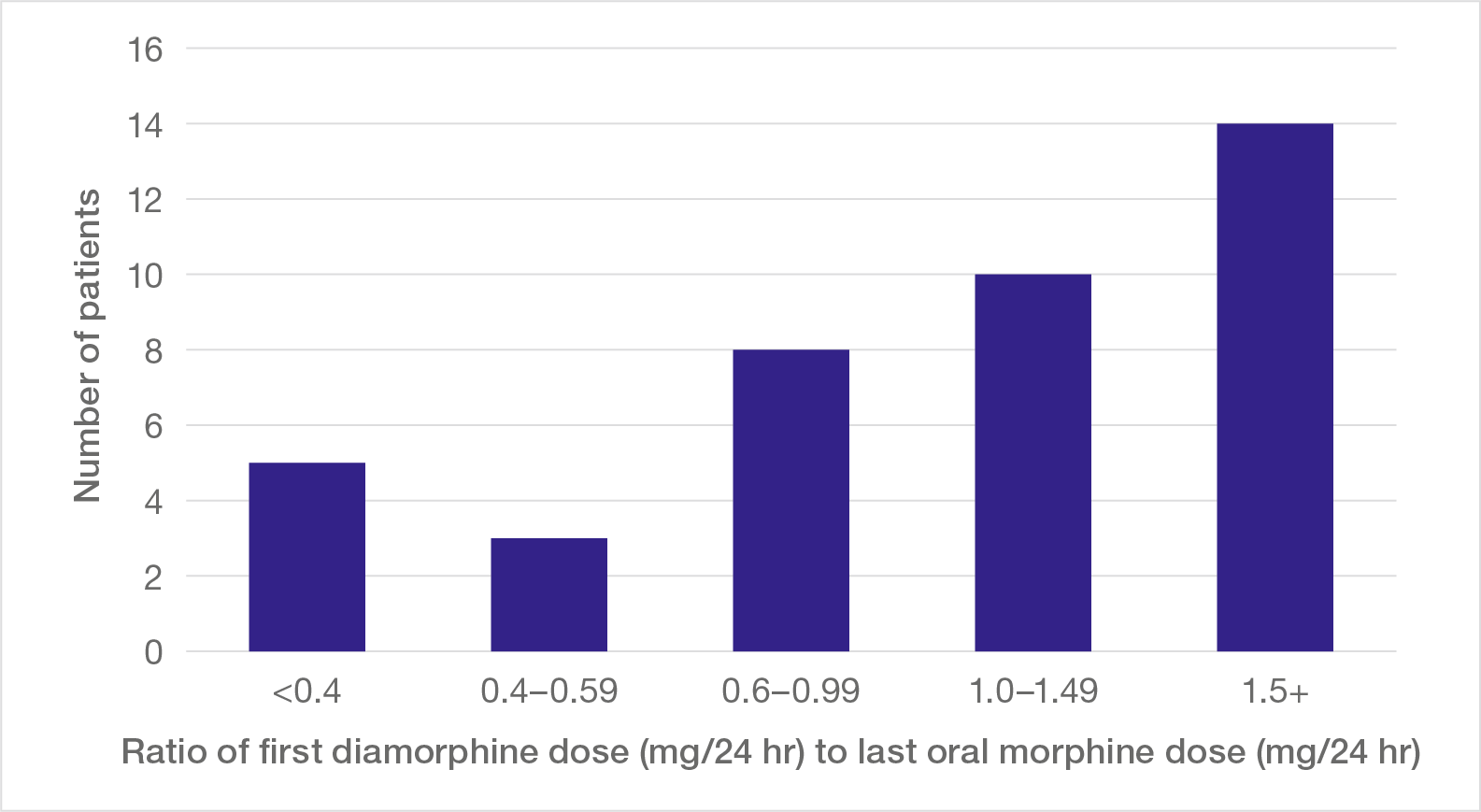
Note: The ratio of first diamorphine dose (mg/24 hr) to last oral morphine dose (mg/24 hr) should usually be within the first bar (<0.4); ratios higher than this contravene guidance.
Finding Five: Opioids combined with other drugs in high doses
Not only were patients started on diamorphine in high doses contrary to the guidance, in many cases diamorphine was combined in the syringe driver with other drugs, most commonly midazolam and hyoscine. As already seen, midazolam is a powerful sedative, used to reduce anxiety, but in concert with diamorphine it acts to suppress consciousness. Hyoscine, used to reduce bronchial and other secretions, also has a suppressive effect on consciousness.
Table 3: Number of patients given diamorphine, midazolam and hyoscine, and all three drugs (percentages of total group)

Table 3 summarises the drugs given via syringe driver to 116 patients from the Initial Group. A large majority received more than one drug, and more than half received all three drugs. The clinical records contain few references to either a need for sedation or signs or symptoms suggesting a need to reduce secretions. Given the high doses of diamorphine prescribed, often without appropriate clinical indication, the addition of further drugs with few references to any clinical requirement for them is remarkable. It is not surprising that excessive levels of sedation were often a feature of what clinical records there were, and it is likely that all three drugs played a part in this when used in combination. It is equally surprising that, considering the amount of opioids being used, there is not a single example in any of the records we have seen where the antidote to opioids, namely naloxone, was considered.
Finding Six: Few patients survived long after starting continuous opioids
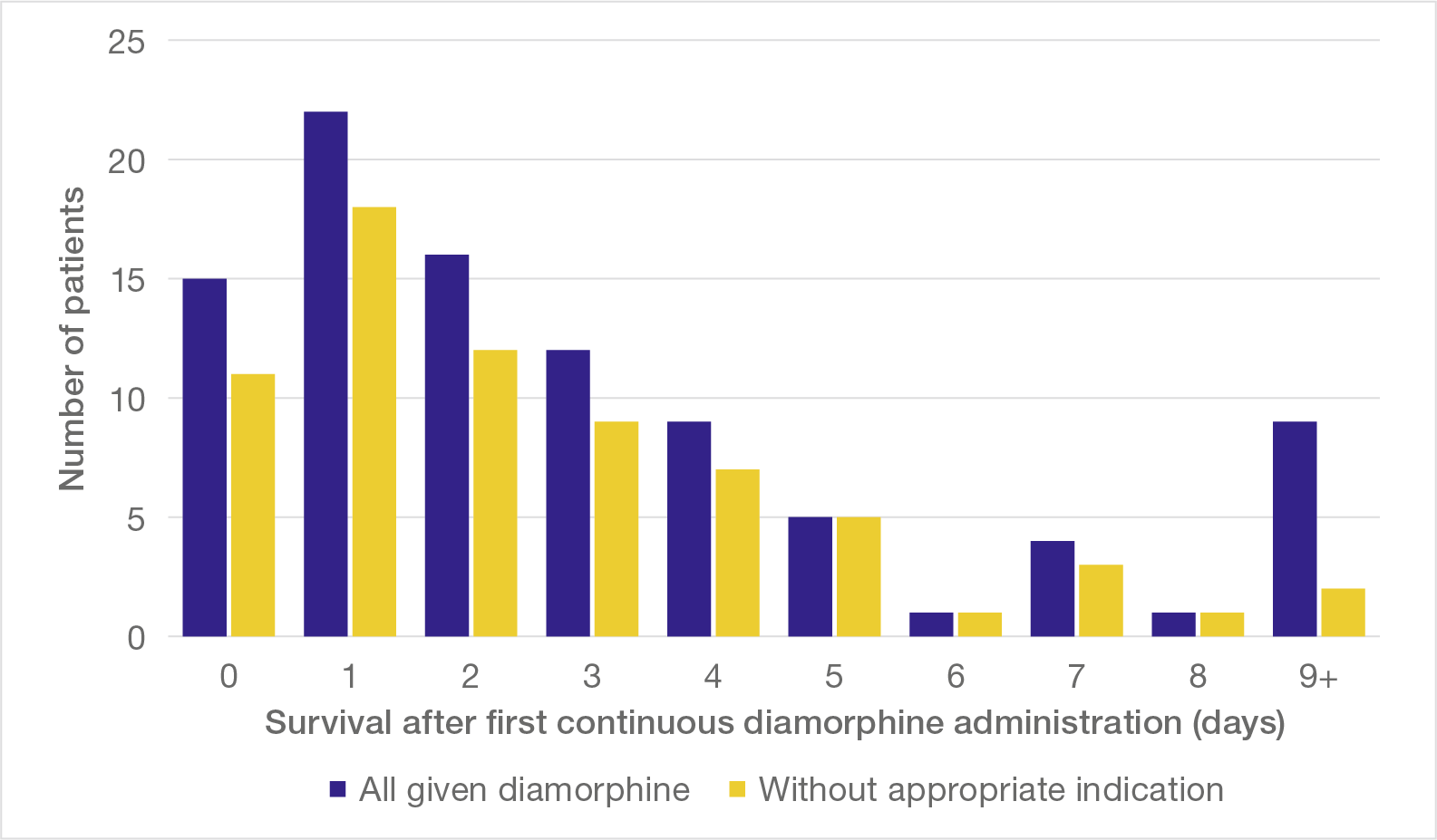
The survival of patients who were started on continuous diamorphine via syringe driver was inevitably measured in days, usually very few. Figure 7 shows the interval between starting diamorphine and death in the Initial Group of patients: 56% survived for two days or less.
Many of these patients were frail and elderly, and some may have been unlikely to survive for a prolonged period; this would be particularly true for those who were given continuous opioids for intractable pain as part of end of life care. Almost three-quarters of this group of patients, however, were given continuous diamorphine without appropriate clinical indication, and it is unlikely that significant numbers of that group would otherwise have succumbed within two days, however frail and elderly.
Figure 7 also shows the survival of those administered continuous diamorphine without appropriate clinical indication (yellow bars). The pattern was at least equally as striking: 59% were dead in two days or less.
Figure 7: Survival after starting continuous diamorphine administration
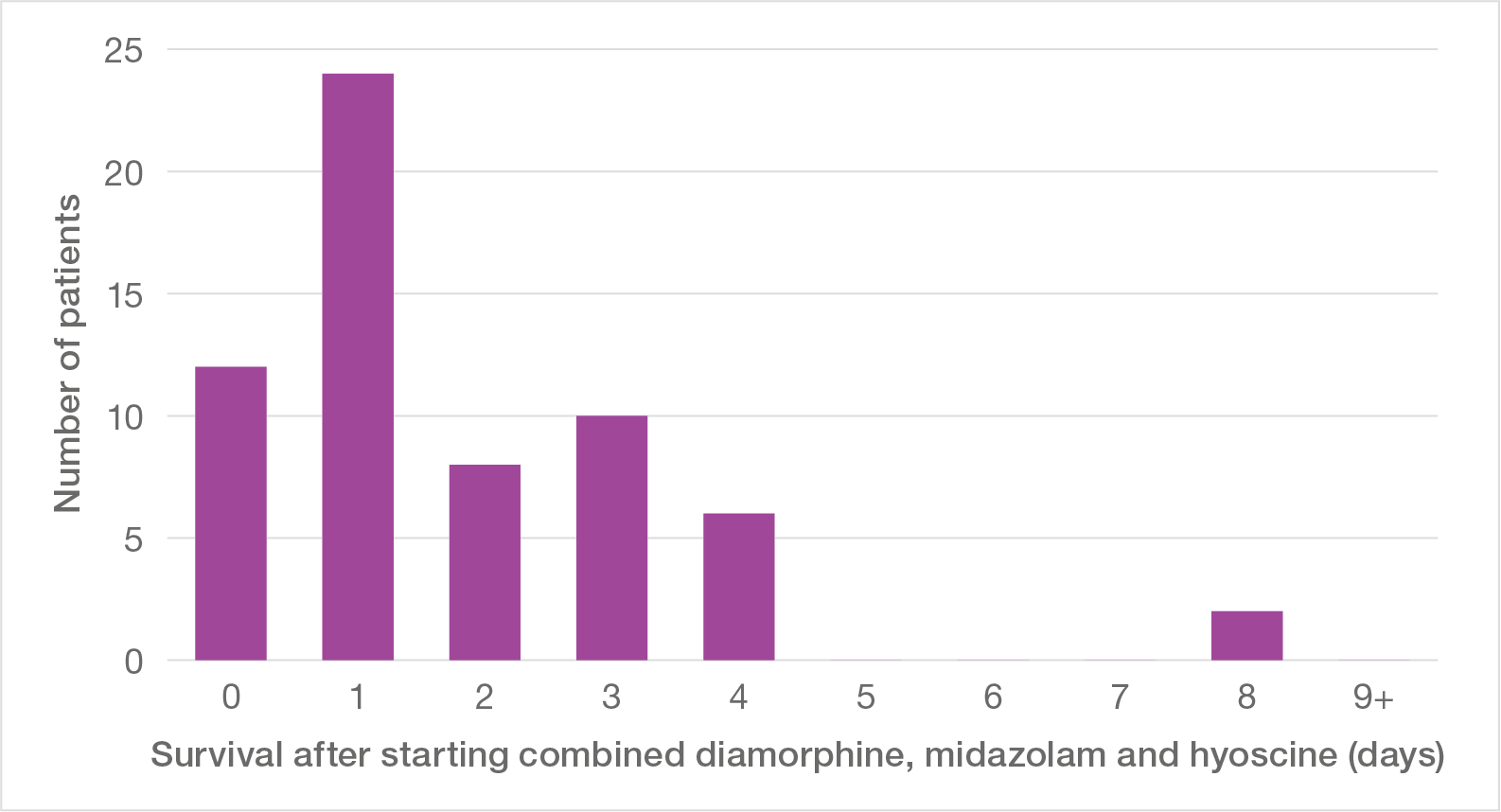
When diamorphine was combined with midazolam and hyoscine, survival was even briefer (Figure 8). Of patients given all three drugs, 71% were dead in two days or less, with half dying on the same or the next day.
Figure 8: Survival after starting combined diamorphine, midazolam and hyoscine
Finding Seven: Prescription and administration of drugs contravened guidelines
The Panel’s analysis shows that the practice of prescribing and administering drugs at the hospital conflicted with the national and local guidance that applied at the time.
Paragraphs 2.87 and 2.106 describe the background to anticipatory prescribing. The failure to follow the relevant guidance extended to record keeping on the wards: the available records show that drug dosage was not always recorded in full on the drug chart. This is further considered in Chapter 3.
Finding Eight: Occurrence and certification of deaths
The Panel retrieved death certificate information for patients who had died in the hospital between the start of 1987 and the end of 2001 – 2,010 in all. The occurrence of deaths followed a striking pattern over time, as shown in Figure 9. Until 1992, the number of deaths per year was fairly steady, around 100 per year, but from 1993 to 1998 the annual death rate rose steadily to reach just over 200 in 1998, double the earlier figure. Equally remarkably, annual deaths fell rapidly over 1999 and 2000 and had reached the former baseline of 100 per year by 2001.
Figure 9: Deaths certified at Gosport War Memorial Hospital, 1987 to 2001
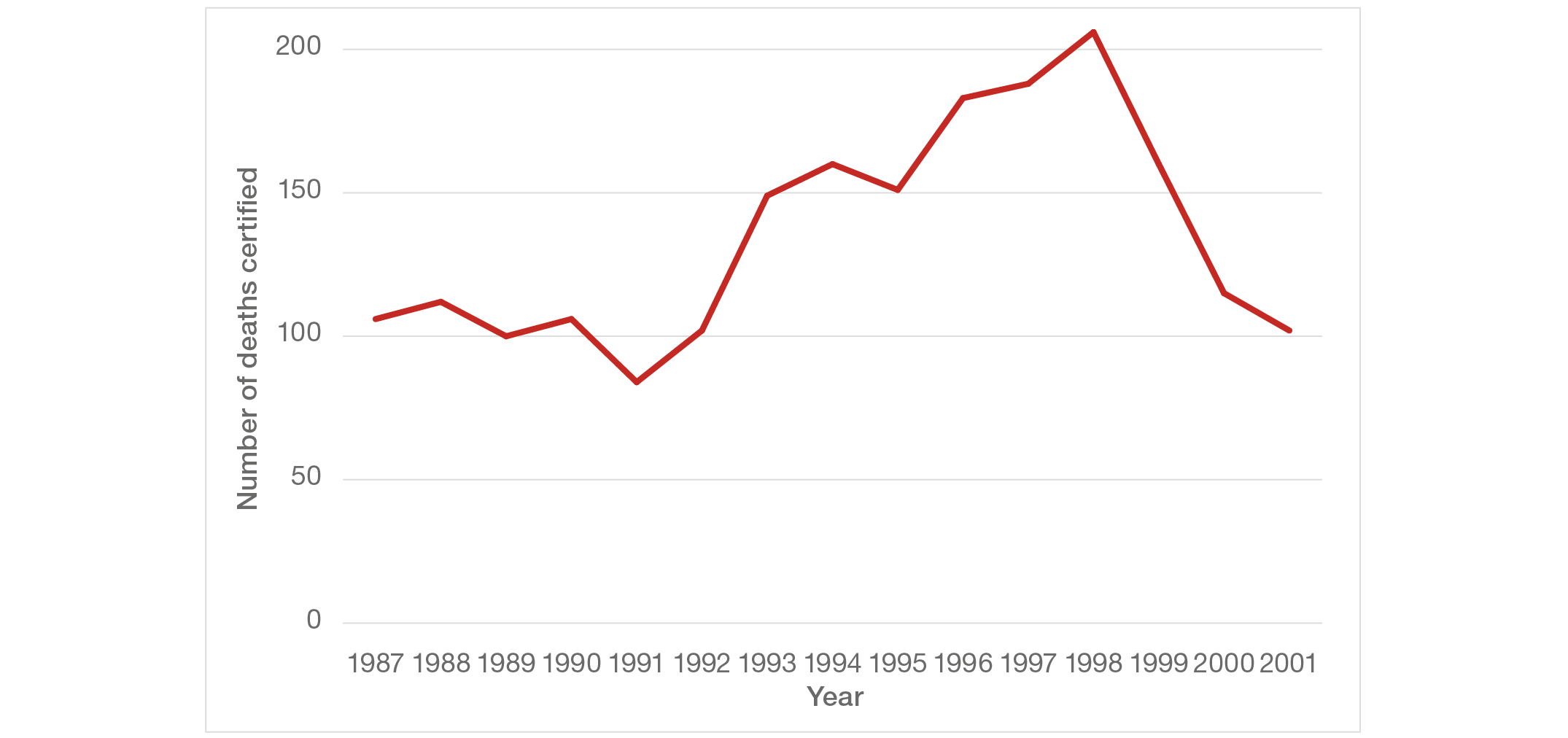
When the rising pattern of deaths was noted previously, two possible explanations were put forward. It was suggested that either the number of patients admitted had increased over the period, or the type of patients had changed, with more admitted for end of life care. There is some evidence that admissions increased, as happened in most hospitals over that period, but information is lacking to test either suggestion directly. Neither suggestion is sufficient to account for the pattern.
First, the rise in deaths was followed by an even more rapid decrease, returning to the previous baseline in two years. To achieve either a reduction in admissions or a change of case-mix sufficient to reverse the rise in two years, a marked change in policy would have had to be implemented in the hospital. There is no recorded evidence of any such policy being considered or implemented.
Second, the rise in deaths corresponds with the time period in which the number of patients given opioids without appropriate clinical indication was at a high level (see Figure 2), whereas an increased need for appropriate opioid administration would have resulted in a steady or decreased number being given opioids without appropriate clinical indication.
The recorded causes of death for those who died in the hospital are also instructive. The most notable feature immediately apparent on examining the death certificates was the frequent occurrence of bronchopneumonia as a cause of death: bronchopneumonia was cited as the immediate cause of death in 796 patients (39%). Bronchopneumonia is a poorly defined infection of the lungs and small airways that may occur as a terminal event in some patients who are very ill, immobile and have reduced consciousness. It is usually accompanied by altered chest sounds and sometimes a raised temperature. Under these circumstances, it is not a cause of death that would generate surprise. Without a clear underlying cause and in the absence of clinical signs, however, it would be an inappropriate cause of death to certify.
Medical certificates of cause of death allow for recording a chain of three direct causes, the first due to or as a consequence of the second, which itself may be due to or as a consequence of the third. The first recorded is the immediate cause of death, while the last completed line is the underlying cause of death; they may be the same if only the first line is completed. There is also scope to record one or two contributory causes which did not themselves directly lead to death. The underlying cause of death was recorded as bronchopneumonia in 503 patients (25%). It is notable that a quarter of all patients who died in the hospital over this period had no other certified cause leading to death than bronchopneumonia, even more so given the lack of clinical findings that would point to this diagnosis in those clinical records examined by the Panel.
Further, the pattern of deaths certified as due to an underlying cause of bronchopneumonia follows broadly the same trend over time as that for all deaths and for the use of opioids without appropriate clinical indication (see Figure 10). This is contrary to the pattern that would have been expected had the rise and subsequent rapid fall in deaths over this period been related to an increase in admissions of more severely ill patients for end of life care. It is also notable that certifying deaths as due to bronchopneumonia was related to opioid usage: in those with evidence of opioid usage without appropriate clinical indication, 30% had bronchopneumonia given as the underlying cause, whereas in those without such evidence the corresponding figure was 11%.
Figure 10: Underlying cause of death certified as bronchopneumonia, 1987 to 2001
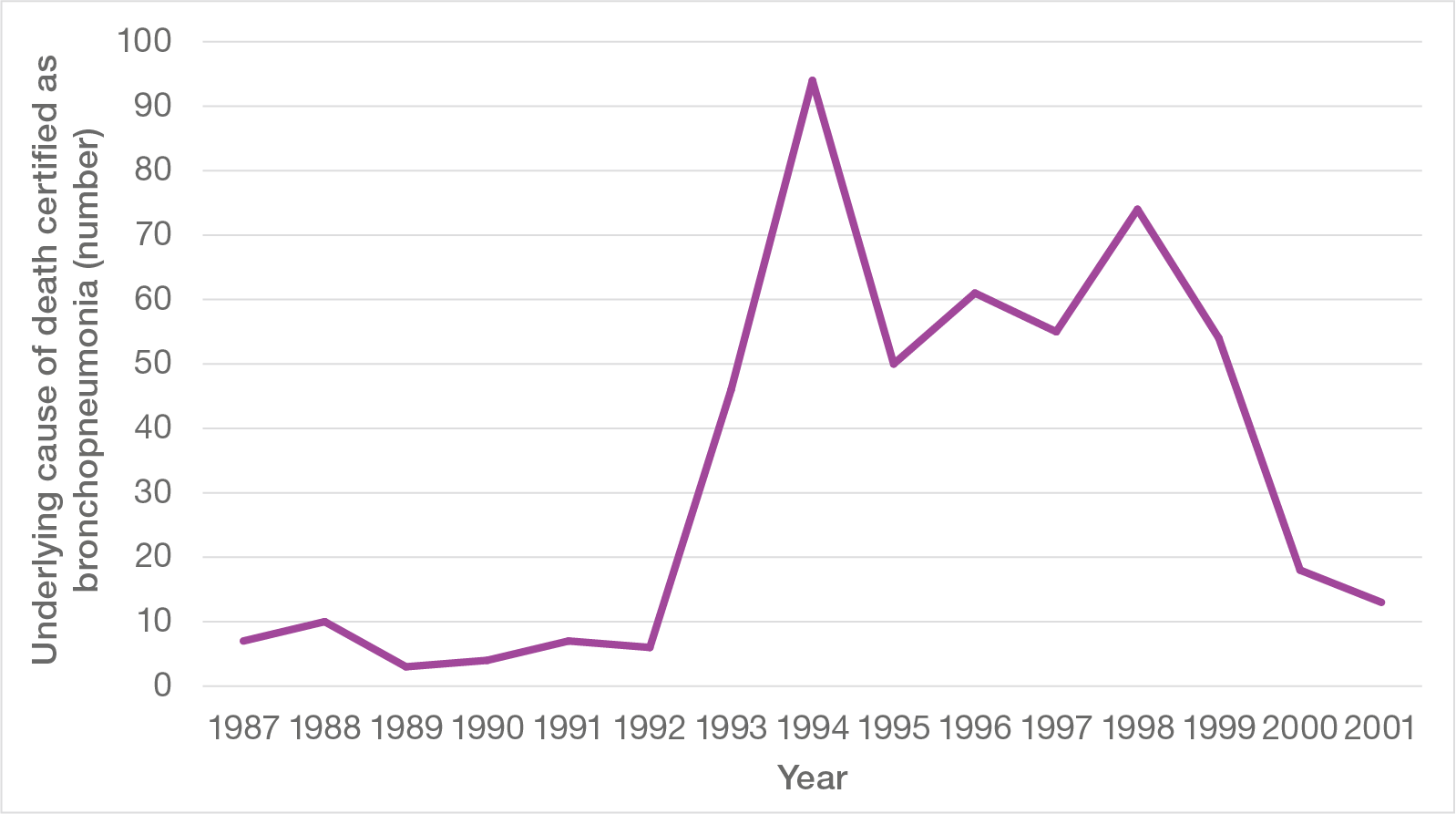
Given the evidence that the rise in deaths over the period 1993 to 2000 was a real increase, it is notable that the number of deaths above those expected from the previous and subsequent baseline amounts to around 500, which is comparable with the 450 to 650 occurrences of use of opioids without appropriate clinical indication described previously.
Finally, it is notable that the overall trend evident in Figure 10, rising from a low baseline in 1992 to a higher plateau from about 1994 to 1998 before falling rapidly back to the baseline in 2001, closely mirrors both the overall number of deaths and the occurrence of diamorphine usage without appropriate clinical indication. These are shown together in Figure 11.
Figure 11: Occurrence of all deaths, opioid use without appropriate clinical indication and certification of bronchopneumonia as underlying cause of death, 1987 to 2001